The Golgi-Cox stained whole mouse brain, using NDT104 FD Rapid Golgistain kit. Note the multiple scans of slide sections, aligned and stitched using Nikon NIS-Elements AR software.
Detection of apoptotic neurons from dorsal ganglion of a mouse embro, with NDT102 FD NeuroApop kit, counterstained with methyl green.
Detection of apoptotic neurons from dorsal ganglion of a mouse embro, with NDT102 FD NeuroApop kit, counterstained with methyl green.
Detection of apoptotic neurons in the striatum of a rat model of stroke, with NDT102 FD NeuroApop kit then counterstained with methyl green
Detection of neurodegeneration with NDT103 FD NeuroSilver Kit in the dentate gyrus of the hippocampus from a rat injected with kainic acid, showing degenerating neurons and processes (black) in the polymorphic layer (pl) and the molecular layer (ml), respectively.
Detection of neurodegeneration in the striatum of a rat brain of a stroke model, demonstrating neuronal damage with NDT103 Kit. Note the accumulation of metallic silver grains (black) in both the striatum and the cortex, indicating the presence of neurodegeneration.
Detection of neurodegeneration in the cortex of an epilepsy animal model. Note degenerating neurons (black) in the deep layers
Detection of neurodegeneration and amyloid plaques in the mouse brain of a model for Alzeimer's disease using NDT 103 Kit. Note silver-stained plaques in both the hippocampus and the cortex.
High mignification of the hippocampal CA1 area as shown above. Note that in addition to silver-stained amyloid plaques, numerous degenerating fibers are also present in the corpus callosum.
Detection of neurodegeneration in the rat brain of a model for Huntington's disease that received a focal injection of QA. The section was processed first for bcl2-immunoreactivity and then for detecting neuronal damage with NDT103 Kit. Note that degenerating neurons (black) intermingle with survived bcl2-positive neurons.
Golgi-Cox impregnation of human cerebral cortex, using NDT104 FD Rapid Golgistain kit.
Golgi-Cox impregnation of rat subiculum, using NDT104 FD Rapid Golgistain kit. Note the various shapes of the spine subtypes (arrow, right).
Detection of cells undergoing apoptosis, by NDT101 FD Apop kit
Comparison of spine density between knockout (upper) and wildtype (lower) mice, using NDT104 FD Rapid Golgistain kit. Note a difference in spine density of dendritic segments.
Golgi-Cox impregnation of purkinje cells of the cerebellum, using NDT104 FD Rapid GolgiStain kit.
Parvalbumin-immunostained section counterstained with thionin. 30-micron cryostat section through the medial entorhinal cortex of a rat that survived for 24 hr after kainic acid administration, showing the preferential loss of neurons in layer III and relative resistance of parvalbumin neurons.
Cytokeratin 18-immunostained section counterstained with cresyl violet. This 12-micron frozen section of the rat prostate was processed for Cytokeratin 18-immunoreactivity (brown) and was then counterstained with NDT202 cresyl violet solution.
Cytokeratin 18-immunostained section counterstained with cresyl violet. This 12-micron frozen section of the rat prostate was processed for Cytokeratin 18-immunoreactivity (brown) and was then counterstained with NDT202 cresyl violet solution.
CD31-immunostained section counterstained with hematoxylin. This 7-micron paraffin section of the mouse ear was processed for CD31-immunoreactivity (brown) and was then counterstained with NDT204 hematoxylin solution.
Hematoxylin (H) & Eosin (E) stain. This 5-micron paraffin section of mouse heart was stained with NDT204 FD hematoxylin (blue) and NDT203 FD eosin Y (red).
The seizure-induced morphological changes of the mouse dentate gyrus, countered stained by NDT106 Rapid TimmStain kit and Nissl.
High magnification of the (inner) molecular layer of the dentate gyrus of seizured brain.
GolgiChrome Kit
Figure 1. Golgi + Tyrosine hydroxylase (TH) immunohistochemistry in the striatum.
Figure 2. Golgi + TH + PSD95 immunohistochemistry in the substantia nigra (SN, upper panel) and anterior commissure (ACA, lower panel).
Figure 2. Golgi + TH + PSD95 immunohistochemistry in the substantia nigra (SN, upper panel) and anterior commissure (ACA, lower panel).
Golgi + TH + PSD95 or SynI immunohistochemistry of the prefrontal prelimbic zone of the rat brain, by Spiga et al (2012).
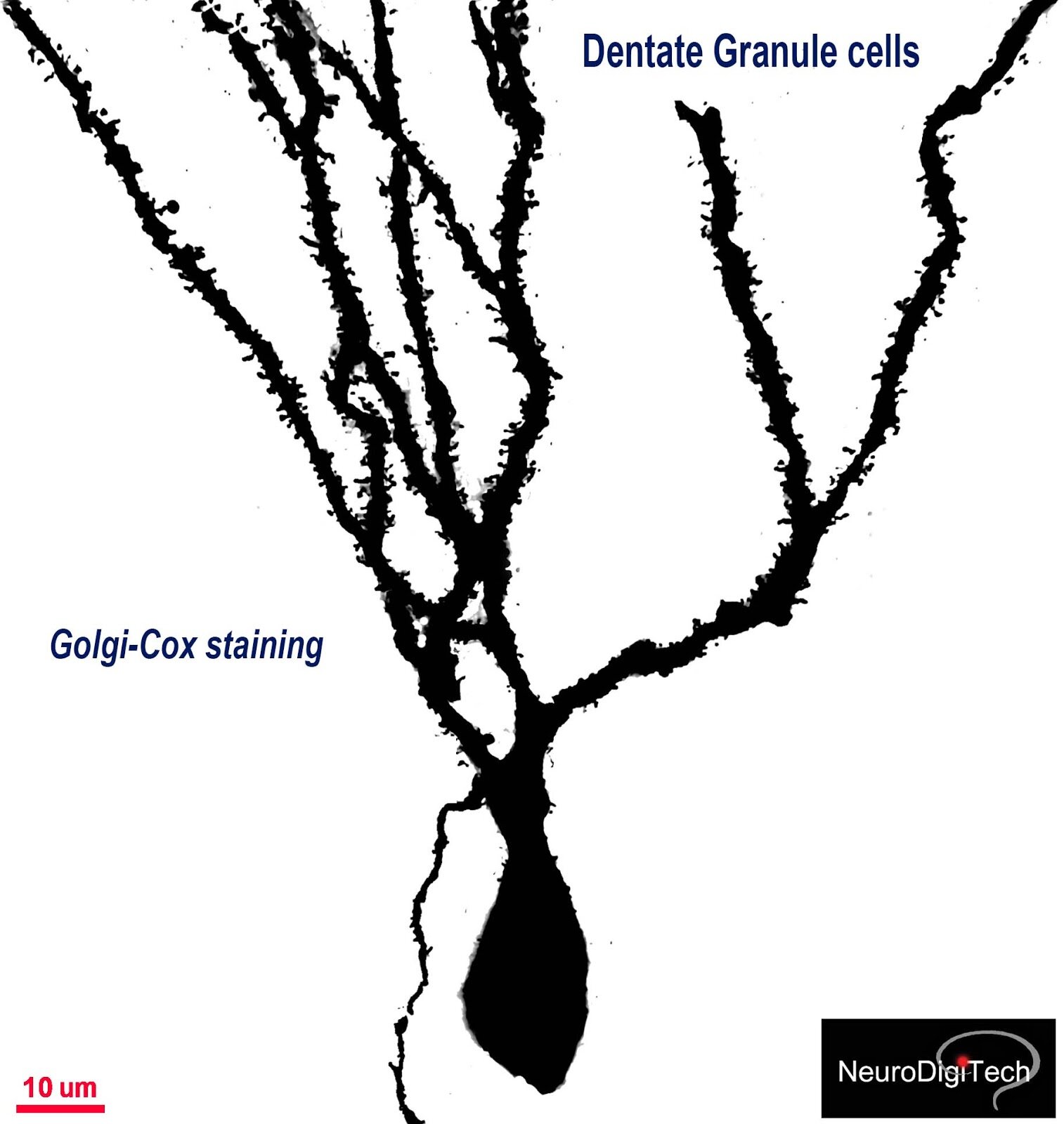
The Golgi Project: 3D morphometrics of Dentate Granule Cells
3D reconstruction of Golgi–Cox impregnated neurons
Golgi-cox stained brain section
NDT206 Neural Red staining of the hippocampal subfields of a human brain
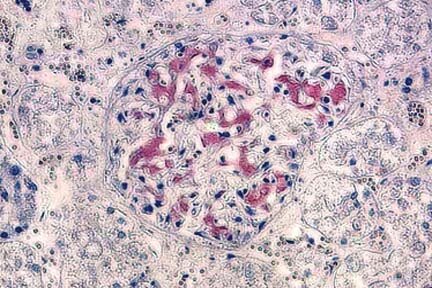
Detection of amyloid deposits (red) and the nuclei will be stained blue in the mouse hippocampus (5-um thick section), by NDT207 Congo Red Solution.
The expression of CR+ cells in CA1 of the hippocampus, stained by Congo Red counterstained with H & E
The spinal cord section stained with NDT208 FD Luxol Fast Blue Solution.
Heat shock protein-immunoreactivity. This 30 µm cryostat section was cut from the dorsal hippocampus of a rat that survived for 15 hrs after the injection of aminooxyacetic acid into the entorhinal cortex (for details, cf. Neurosci. Lett. 147:185-188, 1992). The section was processed free-floating according to avidin-biotin-complex method.
Heat shock protein-immunostained section counterstained with thionin. This 30 µm cryostat section was cut from the dorsal hippocampus of a rat that survived for 15 hrs after the injection of aminooxyacetic acid into the entorhinal cortex (for details, cf. Neurosci. Lett. 147:185-188, 1992). The section was processed for heat shock protein-immunostaining (brown) and then counterstained with thionin.
OX18-immunostained section counterstained with thionin. This 30 µm cryostat section was cut from the entorhinal cortex of a rat that survived for 24 hrs after the injection of aminooxyacetic acid into the entorhinal cortex. The section was processed for OX-immunostaining and then counterstained with thionin. Note the expression of OX-18 immunoreactivity (brown) in activated microglia within layer III.
GFAP-immunostained section counterstained with thionin. This 30 µm cryostat section was cut from the entorhinal cortex of a normal rat. The section was processed for GFAP-immunostaining and then counterstained with FD thionin solution (cf. Products, Cat. #PS101). Note GFAP-immunoreactivity (brown) in the processes of astrocytes.
GFAP-immunostained section counterstained with thionin. This 30 µm cryostat section was cut from the entorhinal cortex of a normal rat. The section was processed for GFAP-immunostaining and then counterstained with FD thionin solution (cf. Products, Cat. #PS101). Note GFAP-immunoreactivity (brown) in the processes of astrocytes.
The colorimetric immunohistochemistry of the moust retina.
GFAP-immunostained section counterstained with thionin. This 30 µm cryostat section was cut from the entorhinal cortex of a normal rat. The section was processed for GFAP-immunostaining and then counterstained with FD thionin solution (cf. Products, Cat. #PS101). Note GFAP-immunoreactivity (brown) in the processes of astrocytes.
GFAP-immunostained section counterstained with thionin. This 30 µm cryostat section was cut from the entorhinal cortex of a normal rat. The section was processed for GFAP-immunostaining and then counterstained with FD thionin solution (cf. Products, Cat. #PS101). Note GFAP-immunoreactivity (brown) in the processes of astrocytes.
Vasoactive intestinal polypeptide-immunofluo-rescence in the spinal cord. A 10 µm cryostat section of the chicken spinal cord was processed on slide according to the indirect fluorescence method. Note 2 large neurons containing vasoactive intestinal polypeptide in the nucleus of the dorsolateral funiculus (for details, cf. J. Comp. Neurol. 278:253-264, 1988).
Tyrosine hydroxylase-immunoreactivity in the rat substantia nigra. 30 µm cryostat section cut coronally from the midbrain of a normal rat. This section was processed free-floating according to the immunogold labeling technique. Note densely labeled neurons in the compact area of the substantia nigra.
Tyrosine hydroxylase-immunoreactivity in the rat substantia nigra. The high magnification of the substantia nigra as shown above. Note that dense black deposits in the cytoplasm of neurons are actually metallic silver grains, which can also be viewed under a microscope with a darkfield condenser.
Bcl2 & parvalbumin double immunostaining. 30 µm cryostat section of the rat cortex was processed free-floating for parvalbumin-immunoreactivity (black deposits) with the immunogold labeling technique and then for bcl2-immunoreactivity according to avidin-biotin-complex method (red).
GABA & parvalbumin double immunostaining. 30 µm cryostat section of the rat cortex was processed free-floating for parvalbumin-immunoreactivity (black deposits) with the immunogold labeling technique and then for GABA-immunoreactivity (red) according to avidin-biotin-complex method. Note that metallic silver grains are accumulated in both parvalbumin-containing neuronal perikarya and processes (probably axon terminals), many of which surrounds GABA neurons.
NeuN & bcl2 double immunostaining. 30 µm cryostat section of the rat cortex was processed free-floating for bcl2-immunoreactivity (black deposits) with the immunogold labeling technique and then for NeuN-immunoreactivity according to avidin-biotin-complex method (red). Note metallic silver grains mainly accumulated in the cytoplasm of bcl2-containing neurons.
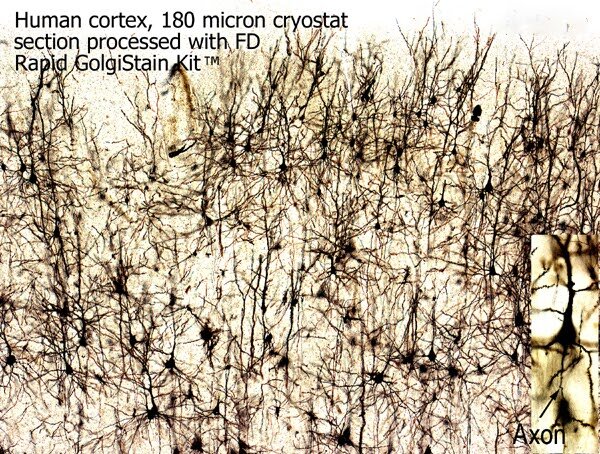
Human cortex, stained by NDT104, Rapid Golgistain kit. Note the details of Golgi-impregnated soma and axons (lower right).
The whole mouse brain, stained by NDT104 FD Rapid GolgiStain kit.
The difference in spine density pf pyramidal cells between KO (knockout) and Wildtype (WT) mice, stained by NDT104, Rapd GolgiStain kit.
GABA & parvalbumin double immunostaining. 30 µm cryostat section of the rat cortex was processed free-floating for parvalbumin-immunoreactivity (black deposits) with the immunogold labeling technique and then for GABA-immunoreactivity (red) according to avidin-biotin-complex method. Note that metallic silver grains are accumulated in both parvalbumin-containing neuronal perikarya and processes (probably axon terminals), many of which surrounds GABA neurons.
Timm’s staining in the dentate gyrus of a rat model for epilepsy. High magnification of the dentate gyrus from a rat that had developed chronic seizures. Note a dense plexus of reaction product in the inner (supragranular) layer of the dentate gyrus.
Timm's staining in the dentate gyrus of a normal rat. High magnification of the polymorphic, granule cell and molecular layers of the dentate gyrus from the same section as shown above . Note little reaction deposits in both the granule cell and molecular layers.
3D reconstruction of the hippocampus (green) of a mouse brain.
Timm’s staining in the dentate gyrus of a rat model for epilepsy. High magnification of the dentate gyrus from a rat that had developed chronic seizures. Note a dense plexus of reaction product in the inner (supragranular) layer of the dentate gyrus.
Multi-Panel digital imaging workstation.
Volumetric analysis using Stereology-based image software program.
Volumetric analysis using Stereology-based image software program.
3D distribution of BrdU+ cells in mouse dentate gyrus
Morphological differences in dendritic morphology of pyramidal cells between WT (Wildtype) and Transgenic (TG) brains.
The dentate gyrus of a mouse brain, stained by NDT104 FD Rapid GolgiStain kit. Note the detate granule cells of DG (dentate gyrus) and Polymorphic cells of the Hilus (H).
3D neuron modeling (click!)
3D neuron reconstruction (click !)
Dendrograms of individual pyramidal cells including the lenght measures of basal and apical dendrites.
Sholl analysis of the basal dendrites of CA1 pyramidal cells
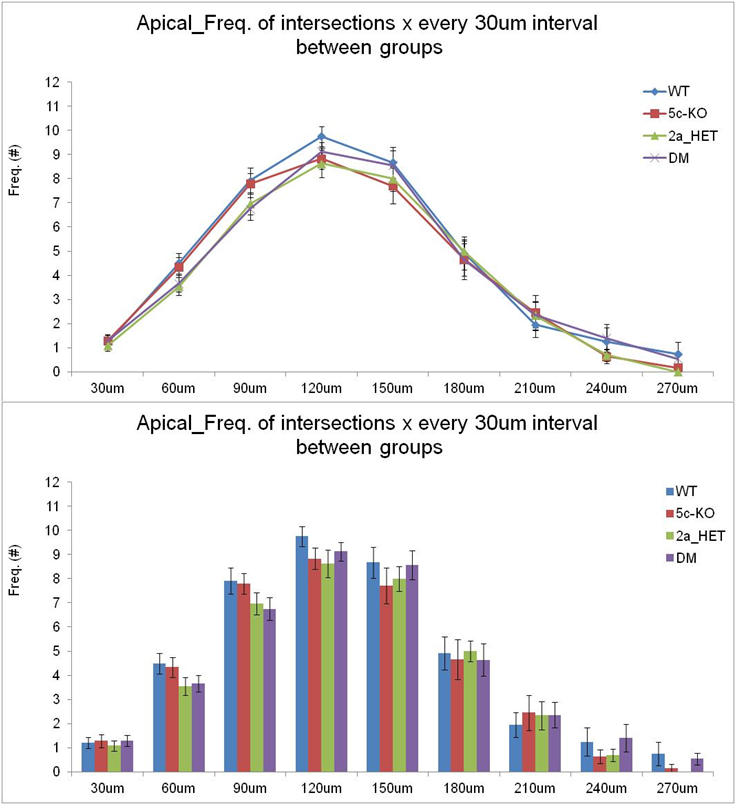
Sholl analysis: Frequency of intersections of basal dendrites of mouse pyramidal cells between groups.
BrdUand NeuN double-immunostaining. 30 £gm cryostat section was cut from the hippocampal dentate gyrus of a mouse sacrificed 24 hr after the injection with 5-bromo-2-deoxyuridine (BrdU). The section was processed free-floating for BrdU-(blue) and then for NeuN-(red) immunoreactivity according to the ABC-AP method (cf. also photo samples of NDT401b).
C-fos and kir2.3 double immunostaining. 30 £gm cryostat section through the rat hypothalamus was processed free-floating for kir2.3- (brown) and then for c-fos- (red) immunoreactivities according to the avidin-biotin-complex method. Note nuclear labeling of c-fos immunoreactivity.
Colocalization of Golgi-Cox, Synapsin 1 and PSD95 of the pyramidal cells of a mouse brain.
The seseizure-induced morphological changes in the detate gyrus and hilus, by NDT106 Rapid TimmStain kit counterstained with Nissl,
A German Göttingen minipig brain (4% paraformaldehyde post-fixed).
Colocalization of Golgi-Cox, PSD95 and Synapsin 1 in the pyramidal cells of mPFC of a mouse brain, usig NDT107 GolgiChrome kit.
Golgi-Cox impregnated CA1 pyramidal cells of the mouse hippocampus using NDT104 Rapid GolgiStain kit.
Golgi-Cox impregnated pyramidal cells in CA1 of the hippocampus of a mouse brain
Golgi-Cox impregnated cells in the ventral posteromedial nucleus of thalamus (VPM) of a mouse brain (inverted image).
Digitized neuron morphology
Dendritic spine quantitation--Golgi Impregnation
3D Neural Morphometrics
3D neuron modeling
Golgi-Cox impregnated dendrites of pyramidal cells crossing the neighboring blood vessels in the mouse cerebral cortex.
Timm-stained sections, counterstained with nuclear stains, such as Nissl to visualize more details of seizure-induced morphological alterations
Double staining of immunoreactivity of Tyrosine Hydroxylase (TH) and Golgi-Cox impregnation, by NDT107 GolgiChrome kit
A GLP study: Immunohistochemistry of grafted human stem cells in the spinal cord of minipigs
Quantitative analysis of dendritic spine of granule cells of the dentate gyrus of the mouse hippocampus, stained by NDT104 Rapid Golgistain kit.
Synaptic contacts of basal dendrites of pyramidal cells and the axon of a neighboring cell of the visual cortex [see box]
Synaptic contacts of basal dendrites of pyramidal cells and the axon of a neighboring cell of CA1 of the mouse hippocampus