Tissue preparation & neurodegeneration detection with FD NeuroSilver Kit (NDT402a):
This service includes tissue preparation, sectioning, silver-staining, mounting, coverslipping and labeling of slides. As a result, you will receive up to 60 silver-stained sections per brain or per tissue block ready for microscopic observations.
Procedure: Following cryoprotection, tissue will be rapidly frozen in isopentane pre-cooled to -70°C. The frozen tissue will be cut on a cryostat and collected in phosphate buffer containing 4% paraformaldehady. Subsequently, sections cut through various levels (or the levels of your choice) will be processed free-floating for the detection of neurons undergoing degeneration with FD NeuroSilver™ Kit.
FD NeuroSilver™ Kit (cf. Products, Cat. #NDT103) is designed for the detection of degenerating neurons in fixed tissue sections of the central nervous system from experimental animals. This kit has been used widely in animal studies under various experimental conditions. The kit has been proven extremely specific and sensitive for the detection of degenerating neuronal somata, axons, and terminals, as well as amyloid plaques in both the brain and spinal cord (see samples below). It is particularly useful for the detection of small numbers of degenerating neurons that may not be demonstrable with routine histopathological techniques. In addition, this kit may be used in combination with immunohistochemistry (see samples below).
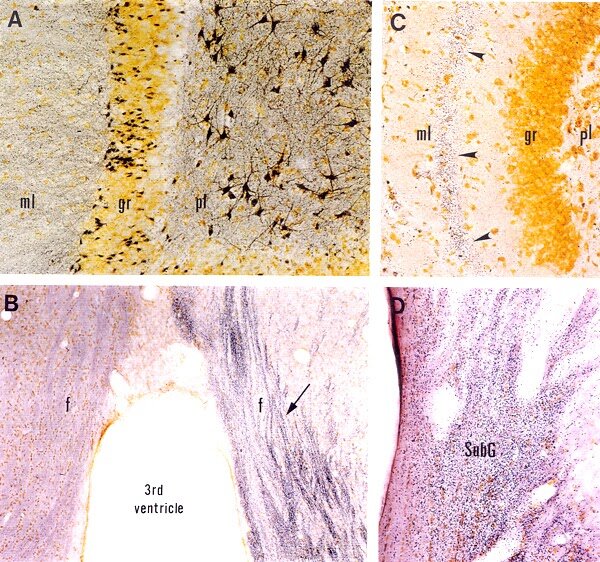
Detection of neurodegeneration with FD NeuroSilver™ Kit I. A: Section (40 µm) through the dentate gyrus of the hippocampus from a rat injected with kainic acid (10 mg/kg, s.c.), showing degenerating neurons and processes (black) in the polymorphic layer (pl) and the molecular layer (ml), respectively. B: Coronal section (30 µm) through the septum of a rat, killed at 10 days following a unilateral transection of the fimbria. Note degenerating axons (indicated by the arrow) in the ipsilateral fornix. C: Horizontal section (40 µm) through the hippocampal dentate gyrus of a rat killed at 5 days after the intra-entorhinal injection with aminooxyacetic acid. Note numerous degenerating axon terminals (arrowheads) in the middle zone of the molecular layer (ml), the terminal field of entorhinal neurons killed by the drug injection (for details, cf. Neuroscience 82:1165, 1998). D: Coronal section (40 µm) throught the lateral geniculate nucleus of a rat killed at 5 days after a unilateral visual cortex aspiration. Note dense degenerating axon terminals in the subgeniculate nucleus (SubG). Section courtesy of Dr. E.-Y. Chen, Rush University (D).
Detection of neurodegeneration in the rat brain of a stroke model. 40 µm cryostat section was cut coronally through the striatum of a rat used as an animal model of stroke. The section was processed for demonstrating neuronal damage with FD NeuroSilver™ Kit. Note the accumulation of metallic silver grains (black) in both the striatum and the cortex, indicating the presence of neurodegeneration.
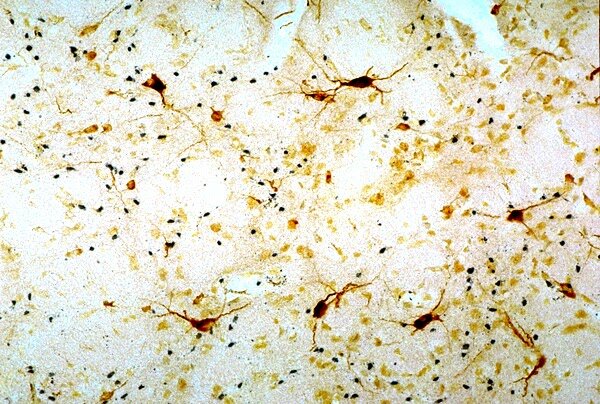
Detection of neurodegeneration in the rat brain of a model for Huntington’s disease. 40 µm cryostat section was cut coronally through the striatum of a rat that received a focal injection of quinolinic acid. The section was processed first for bcl2-immunoreactivity and then for detecting neuronal damage with FD NeuroSilver™ Kit I. Note that degenerating neurons (black) intermingle with survived bcl2-positive neurons.
Detection of small numbers of degenerating neurons by NDT103 in combination with the immunohistochemistry.
Remarks:
A quotation is required before placing an order.
The investigator needs to provide fixed tissue.
Please contact us for more information.
Tissue preparation & neurodegeneration detection with Gallyas silver staining technique (NDT402b):
This service includes tissue preparation, sectioning, silver-staining, mounting, coverslipping and labeling of slides. As a result, you will receive up to 40 silver-stained sections per brain or per tissue block ready for microscopic observations.
Procedure: Following cryoprotection, tissue will be rapidly frozen in isopentane pre-cooled to -70°C. The frozen tissue will then be cut on a cryostat and collected in our unique section cryoprotection solution (cf. Products, Cat. #NDT301). Subsequently, sections cut through various levels (or the levels of your choice) will be processed free-floating for the detection of neurons undergoing degeneration with Gallyas silver staining technique¹.
This technique was originally described by Gallyas et al.¹ and later modified². It is particularly useful for the detection of early neuronal injury in the central nervous system of experimental animals. Typically, both the neuronal perikarya and processes are silver-stained. This technique permits the morphological categorization of damaged neurons and the detection of subtle changes in the morphology of cell bodies and processes (cf. see samples below)
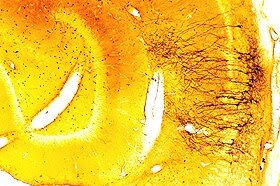
Gallyas method_Detection of early neuronal injury in the cerebral cortex.
Degenerating dendrites of pyramidal cells in the cerebral cortex.
Remarks:
A quotation is required before placing an order.
The investigator needs to provide tissue fixed by perfusion with a special fixative.
Please contact us for more information.
References:
Gallyas et al. (1990) Acta Neuropath. 79, 620-628.
Du et al. (1998) Neuroscience 82, 1165-1178.
Tissue preparation & neurodegeneration detection with in situ nick translation technique (ISNT) (NDT402c):
This service includes sectioning, ISNT labeling, coverslipping and labeling of slides. As a result, you will receive up to 40 ISNT-labeled sections per brain or per tissue block ready for microscopic observations.
Procedure: Unfixed frozen tissue will be cut on a cryostat and mounted on proteinase-resistant microscope slides (Slides available upon request). Sections cut through various levels (or the levels of your choice) will be processed on slides for the detection of neurodegeneration with in situ nick translation technique¹.
This technique, originally described by Gold et al. (1993)¹, labels the nuclei of neurons undergoing DNA fragmentation. This method is based on the ability of DNA polymerase I to catalyze the polymerization of deoxyuridines onto the ends of DNA fragments. The integrated biotins are amplified and visualized by the avidin-biotin-complex methods² (cf. see samples below).
The detection of the nuclei of neurons undergoing DNA fragmentation, by ISNT.
Remarks:
A quotation is required before placing an order.
The investigator needs to provide unfixed frozen tissue.
Please contact us for more information.
References:
Gold R., Schmied M., Rothe G., Zischler H., Breitschopf H., Wekerle H. and Lassmann H. (1993) Detection of DNA fragmentation in apoptosis: application of in situ nick translation to cell culture systems and tissue sections. J. Histochem. Cytochem. 41: 1023-1030.
Hsu S. M., Raine L. and Fanger H. (1981) Use of avidin-biotin-peroxidase complex (ABC) in immunoperoxidase techniques: a comparison between ABC and unlabeled antibody (PAP) procedures. J. Histochem. Cytochem. 29: 577-580.
Tissue preparation & neuronal apoptosis detection with in situ DNA nick end-labeling technique (TUNEL) (NDT402d:):
This service includes sectioning, TUNEL labeling, coverslipping and labeling of slides. As a result, you will receive up to 40 TUNEL-labeled sections per brain or per tissue block ready for microscopic observations.
Procedure: Unfixed frozen tissue will be cut on a cryostat and mounted on proteinase-resistant microscope slides (Slides available upon request). Sections cut through various levels (or the levels of your choice) will then be processed on slides for the detection of neurons undergoing apoptosis with in situDNA nick end-labeling¹.
This technique, originally described by Gavrieli et al. (1992)¹, labels the nuclei of neurons undergoing DNA fragmentation. This method is based on the ability of terminal deoxynucleotidyl transferase to catalyze incorporation of biotinylated deoxyuridines onto the free 3'-hydroxyl termini of DNA fragments, which are considered as one of the most characteristic features of apoptosis², ³. The integrated biotins are amplified and visualized by the avidin-biotin-complex (ABC) method4 (cf. see samples below).
Detection of apoptotic neurons with FD NeuroApop™ Kit. Paraffin section (10µm) cut from a dorsal ganglion of a mouse embryo (E17). The section was processed with FD NeuroApop™ kit and counterstained with methyl green. Sections courtesy of Drs. Michael Vogel and Lisa Qiu.
Detection of apoptotic neurons with FD NeuroApop™ Kit. Paraffin section (10µm) cut from the inferior colliculus of a mouse embryo (E17). The section was processed with FD NeuroApop™ kit and counterstained with methyl green. Sections courtesy of Drs. Michael Vogel and Lisa Qiu.
Detection of apoptotic neurons in a rat model of stroke. 20 µm cryostat section was cut from the rat striatum of a stroke model. The section was processed for detecting neuronal apoptosis with FD NeuroApop™ Kit and then counterstained with FD methyl gree
Remarks:
A quotation is required before placing an order.
The investigator needs to provide unfixed frozen tissue.
Please contact us for more information.
References:
Gavrieli Y, Sherman Y, and Ben-Sasson SA: Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J. Cell Biol. 119: 493-501, 1992.
Wyllie A. H. (1980) Glucocorticoid-induced thymocyte apoptosis is associated with endogenous endonuclease activation. Nature 284: 555-556.
Arends M. J., Morris R. G. and Wyllie A. H. (1990) Apoptosis: the role of the endonuclease. Amer. J. Pathol. 136: 593-608.
Hsu S. M., Raine L. and Fanger H. (1981) Use of avidin-biotin-peroxidase complex (ABC) in immunoperoxidase techniques: a comparison between ABC and unlabeled antibody (PAP) procedures. J. Histochem. Cytochem. 29: 577-580.
Terms and Conditions
For quality assurance of our service, it is recommended that you discuss with us for preferred perfusion protocol and histology and/or immunolabeling protocols.
It is suggested that you use Gel-coated microscopic slides for tissue mounting and 0.17um-thick coverslips.
A 15% of the fee will be due upon authorization of the study; and the remaining fee will be due upon delivery of study results.
Progress of the service is contingent upon staining quality of tissues, operated by the independent contractor.
Should early termination occur, Neurodigitech will prorate the cost incurred and invoice the Sponsor. The first portion of the fee is non-refundable.